| CAS
NO. |
1098-97-1 (parent),
10049-83-9 (dihydrochloride) |
|
| EINECS
NO. |
214-150-1
(parent), 233-178-5 (dihydrochloride) |
| FORMULA |
C16H20N2O4S2·2HCl |
| MOL
WT. |
441.39 |
|
H.S.
CODE
|
|
|
TOXICITY
|
|
|
PRICE
|
U$1,500
for 1kg
|
| SYNONYMS |
Bonifen; Dinerfene;
Encephabol; Enerbol; |
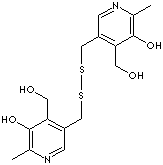
| 3,3'-Dithiobis(methylene)bis(5-hydroxy-6-methyl-4-pyridinemethanol)
dihydrochloride; Pyridoxin-5'-disulfide dihydrochloride; Pyrithioxin; Pyrithioxine;
Pyrithioxine hydrochloride; Pyritinol HCl; Pyritioxine Hydrochloride;
5-[[5-hydroxy-4-(hydroxymethyl)-6-methylpyridin-3-yl]methyldisulfanylmethyl]-
4-(hydroxymethyl)-2-methylpyridin-3-ol
dihydrochloride |
| DERIVATION |
|
|
CLASSIFICATION
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
white crystalline
powder |
| MELTING POINT |
132
- 135 C |
| BOILING
POINT |
|
| SPECIFIC GRAVITY |
|
| SOLUBILITY
IN WATER |
|
| pH |
|
| VAPOR DENSITY |
|
|
AUTOIGNITION
|
|
|
NFPA
RATINGS
|
|
|
REFRACTIVE
INDEX
|
|
| FLASH
POINT |
|
| STABILITY |
Stable
under ordinary conditions |
|
GENERAL
DESCRIPTION & APPLICATIONS
|
|
The effect of a nootropic, Pyritinol, on the recovery of cortical cholinergic
deficits induced by injury of the nucleus basalis has been tested on two groups
of unilateral quisqualic acid nbM-lesioned rats. The first group had a 30 nmol
lesion producing a cortical cholinergic impairment at 21 days, with a
spontaneous recovery at 45 days. The second group had a 50 nmol lesion that
produced a deeper cholinergic deficit, which did not recover at 45 days.
Pyritinol enhanced the recovery in the 30 nmol group of animals on the 21st day
after surgery. The recovery was measured as an increase in the activities of
acetylcholinesterase (AChE), choline acetyltransferase (ChAT) and the high
affinity choline uptake system, and the histochemical densities of the cortical
AChE network and the M2 receptor. Histochemical analysis of the nbM enabled
cortical recovery to be related to the number of surviving neurons and also to
their hypertrophy and AChE-ChAT hyperactivity. Pyritinol enhanced recovery in 30
nmol lesioned animals but in the other group, with a lower number of surviving
neurons and a lower ability of the cells to become hypertrophic, the drug was
unable to promote cortical recovery.
(source: http://www.raysahelian.com/) Pyritinol is a
pyridoxine class compound. Two pyridoxines are linked together by
two sulfur atoms. Pyritinol (or Pyrithioxin) is a nootropic which enhances oxygen and glucose uptake in the brain.
It is also an antioxidant which
scavenges hydroxyl radicals. But it has no vitamin B6 activity.
|
| SALES
SPECIFICATION |
|
APPEARANCE
|
white crystalline
powder |
|
ASSAY
|
97.0
- 103.0% (anhydrous basis)
|
|
WATER
|
5.0%
max
|
|
MELTING POINT |
132
- 135 C
|
| TRANSPORTATION |
| PACKING |
|
| HAZARD CLASS |
|
| UN
NO. |
|
| OTHER
INFORMATION |
|
|
